Background
DNA-hypomethylating agents (HMAs) have improved the prognosis of patients (pts) with acute myeloid leukemia (AML) unfit for standard induction chemotherapy. AML pts aged >60 years eligible for allografting (HCT) can be effectively bridged to HCT by HMA monotherapy. This was demonstrated in the EORTC randomized phase III trial AML21 (NCT02172872) comparing 10-day decitabine (DEC) to “7+3” induction (Lübbert et al., EHA 2022, S125), with similar transplant rates and post-HCT outcomes in both arms. Also the combination of an HMA with venetoclax (VEN) is not only effective in pts unfit for more intensive treatment (DiNardo et al., New Engl. J. Med. 2020) but also in pts fit for HCT (Pollyea et al., BMT 2021, Maiti et al., ASH 2022, #7725). A recent analysis demonstrated that HMA+VEN as pretransplantation therapy can attain post-HCT outcomes comparable to intensive chemotherapy regimens (Winters et al., TCT 2022).
Patients and Methods
We retrospectively analyzed 47 consecutive older AML pts receiving firstline treatment with DEC+VEN. DEC was administered on five consecutive days per cycle. VEN was administered up to 28 days per cycle (see Table 1). All pts who had received at least one dose of DEC and VEN were included. Data on all pts were retrospectively collected as reported in the medical charts. Written informed consent of all pts to analysis of their records for scientific purposes was provided. Key parameters captured and analyzed were: age, ECOG performance status (PS), ELN 2017 risk, number of cycles of DEC+VEN administered, response rates and overall survival (OS). Common safety parameters (e.g., prolonged neutropenia, tumor lysis syndrome, etc.) were assessed.
Results
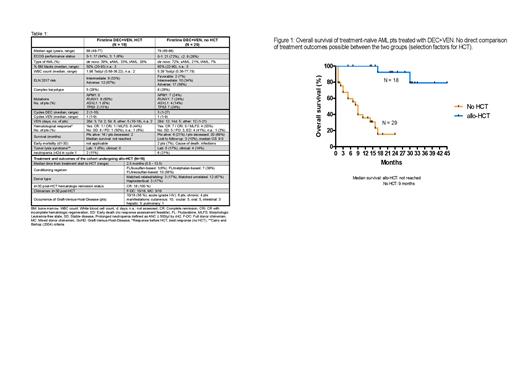
Between 2/2019 and 2/2023, 47 pts with AML received DEC+VEN as firstline treatment. Of those, 18 pts (38%) proceeded to HCT, 29 (62%) did not (ineligibility for HCT, wish of pt, intercurrent events etc.). Median follow-up was 25.0 (HCT) and 14.8 months (no HCT). Key pt characteristics in the two groups were i. median age: 69 and 79 years, ii. ECOG PS 0: 50 and 27%, iii. de novo AML: 39 and 72%, iv. ELN 2017 adverse risk: 67 and 59%, median number of treatment cycles administered were 2.0 and 3.0. Safety data are shown in Table 1. A hematologic response before HCT (CR, CRi, MLFS) was attained in 8/18 pts (44%), with only 2/18 receiving >2 cycles. Median time from treatment start to HCT was 2.5 months (for transplant details, refer to Table 1). For non-transplanted pts, CR, CRi, MLFS as best response were attained in 16/29 (55%), 13/29 receiving >4 cycles. In pts receiving HCT, graft-versus-host-disease occurred in 10/18 pts (56%), median OS was not reached (16/18 pts alive; Fig. 1). In pts not receiving HCT, median OS was 9.0 months. A direct comparison of treatment outcomes between the two groups is not possible, given the different selection factors operative for pts attaining HCT or not.
Conclusions
Among these older AML pts with non-favorable genetics, administration of a limited number of DEC+VEN cycles followed by HCT was well feasible in almost 40% of pts (median age 69 years) and resulted in very encouraging post-HCT OS in this first analysis. In pts not able to receive HCT (median age 79 years), DEC+VEN resulted in response rates, treatment continuation and OS comparable to “real-world” older AML pts treated with AZA+VEN, suggesting equivalent efficacy of both regimes.
Disclosures
La Rosée:Novartis: Research Funding; MSD: Research Funding. Becker:AbbVie: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pierre Fabre Pharma: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Wäsch:Amgen: Consultancy; BMS/Celgene: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Kite/Gilead: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; Janssen: Research Funding; Sanofi: Research Funding; Abbvie: Honoraria; Amgen: Honoraria; BMS/Celgene: Honoraria; Janssen: Honoraria; Kite/Gilead: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Janssen: Other: Travel support; Kite/Gilead: Other: Travel support; Pfizer: Other: Travel support; BMS: Other: Travel support; Sanofi: Honoraria. Finke:Medac: Honoraria, Research Funding; Neovii: Honoraria, Research Funding, Speakers Bureau; Riemser: Honoraria, Research Funding, Speakers Bureau; Gilead Sciences: Current holder of stock options in a privately-held company; AbbVie: Current holder of stock options in a privately-held company; Roche: Current holder of stock options in a privately-held company. Lübbert:AbbVie: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Imago BIosciences: Other: study drug; Cheplapharm: Other: Study drug; Janssen-Cilag: Research Funding; Astex Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal